RESEARCH PAPER
The popularity of the 5- and 6-component combination vaccines among infants' parents in a selected Primary Health Care Clinic
1
Doctoral Studies, Department of Medical Science, Medical University of Silesia, Katowice, Poland
2
Department of Clinical Nursing, Nursing and Midwifery Institute, Collegium Medium, Faculty of Health Sciences, Jagiellonian University, Kraków, Poland
Corresponding author
Marcelina Anna Podstawa
Zakład Pielęgniarstwa Klinicznego, Instytut Pielęgniarstwa i Położnictwa, Wydział Nauk o Zdrowiu, Uniwersytet Jagielloński Collegium Medicum, Polska, ul. Kopernika 25, 31-501, Kraków, Polska
Zakład Pielęgniarstwa Klinicznego, Instytut Pielęgniarstwa i Położnictwa, Wydział Nauk o Zdrowiu, Uniwersytet Jagielloński Collegium Medicum, Polska, ul. Kopernika 25, 31-501, Kraków, Polska
Med Srod. 2020;23(1-4):1-4
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
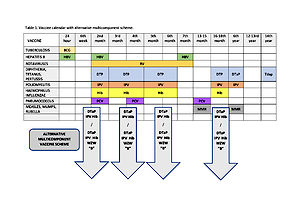
Vaccinations as one of the highest achievements of medicine contributed to a significant reduction of infectious diseases. According to the current vaccination calendar in Poland, parent have possibility to choose free cost classic (DTP, polio, HBV) and not refundable 5- or 6-component combination vaccine. The aim of the study was to assess the popularity of the 5- and 6-component combination vaccines in years 2010-2019 among infants' parents starting a mandatory vaccination series in a selected Primary Health Care Clinic.
Material and methods:
The study was conducted at Med-All Medical Centre in Krakow which provided Protective Vaccination Program and included 1108 immunization cards of children born between 2010-2019. Most of the sample (98.5%) lived in Cracow. This retrospective ecological study was used analysis of medical records. Data analysis MS Excel (Microsoft) and PQStat Soft. The significance level was p< 0.05.
Results:
From 2010 to 2016, 5-component combination vaccine was the most popular choice for infants. During the study period the 5-component combination vaccine usage decrease with years (p=0.017). From 2017, the most popular was the 6-component vaccine and the usage increase with years with strong statistically significance (p=0.005). In 2019 there was the largest percentage of unvaccinated infants.
Conclusions:
Despite the costs, in 2010-2016, the most popular vaccine for infants was the 5-component high-combination vaccine. Since 2017, the 6-component vaccine has gained the greatest popularity.
Vaccinations as one of the highest achievements of medicine contributed to a significant reduction of infectious diseases. According to the current vaccination calendar in Poland, parent have possibility to choose free cost classic (DTP, polio, HBV) and not refundable 5- or 6-component combination vaccine. The aim of the study was to assess the popularity of the 5- and 6-component combination vaccines in years 2010-2019 among infants' parents starting a mandatory vaccination series in a selected Primary Health Care Clinic.
Material and methods:
The study was conducted at Med-All Medical Centre in Krakow which provided Protective Vaccination Program and included 1108 immunization cards of children born between 2010-2019. Most of the sample (98.5%) lived in Cracow. This retrospective ecological study was used analysis of medical records. Data analysis MS Excel (Microsoft) and PQStat Soft. The significance level was p< 0.05.
Results:
From 2010 to 2016, 5-component combination vaccine was the most popular choice for infants. During the study period the 5-component combination vaccine usage decrease with years (p=0.017). From 2017, the most popular was the 6-component vaccine and the usage increase with years with strong statistically significance (p=0.005). In 2019 there was the largest percentage of unvaccinated infants.
Conclusions:
Despite the costs, in 2010-2016, the most popular vaccine for infants was the 5-component high-combination vaccine. Since 2017, the 6-component vaccine has gained the greatest popularity.
REFERENCES (21)
1.
Gołąb J, Jakóbisiak M, Lasek W, et al. Immunologia. Warszawa: Wydawnictwo naukowe PWN; 2017. p. 312–326.
2.
Male D, Brostoff J, Roth D B, et al. Immunologia. Warszawa: Elsevier Urban & Partner; 2008. p. 325–339.
3.
European Medicines Agency gives first opinion for a vaccine for use outside the EU. European Medicines Agency (EMA). https://www.ema.europa.eu/en/n... [accessed 29.04.2021].
4.
Hexacima EPAR. European Medicines Agency (EMA). https://www.ema.europa.eu/en/m... [accessed 29.04.2021].
5.
Hexyon EPAR. European Medicines Agency (EMA). https://www.ema.europa.eu/en/m... [accessed 29.04.2021].
6.
Vaxelis EPAR. European Medicines Agency (EMA). https://www.ema.europa.eu/en/m... [accessed 29.04.2021].
7.
Program Szczepień Ochronnych w 2020 roku. https://szczepienia.pzh.gov.pl... [accessed 10.02.2020].
8.
Wysocki J, Czajka H. Szczepienia w pytaniach i odpowiedziach. Kraków: Help-Med; 2018. p. 133–150.
9.
Dolhain J, Janssens W, Sohn W-Y, et al. Integration of hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliomyelitis and Haemophilus influenzae type b conjugate vaccine within existing national recommendations following a birth dose of monovalent hepatitis B virus vaccine: results of a systematic review in the Asia Pacific region. Expert Rev Vaccines 2019; 18: 921–933.
10.
Kramarz P, Pędziński B, Giesecke J. Tiomersal jako składnik szczepionek. Aktualny stan wiedzy. Med Prakt Pediatr. 2010; 1: 5–12, 7.
11.
Zawadka M, Lutyńska A. Immunogenność i odczynowość bez komórkowych szczepionek przeciw krztuścowi przeznaczonych dla młodzieży i osób dorosłych. Przegl Epidemiol. 2012; 66: 99–105.
12.
Szymoniak K, Cholewa D, Fryc D, et al. Opinia rodziców na temat odmowy wykonania szczepień ochronnych u dzieci. Piel Pol. 2020; 77 (3): 159–165.
13.
Koperny M, Kargul A, Seweryn M, et al. The prevalence of combination vaccines for children in Europe. Analysis of the availability and funding. JHPOR 2014; 2: 18–31.
14.
Monge S, Hahné S J, de Melker H E, et al. Effectiveness of the DTPa-HBV-IPV/Hib vaccine against invasive Haemophilus influenzae type b disease in the Netherlands (2003–16): a casecontrol study. The Lancet Inf Dis. 2018; 18: 749–757.
15.
Pieszka M, Waksmańska W, Woś H. Wiedza rodziców dzieci do drugiego roku życia na temat szczepień ochronnych. Med Og Nauk Zdr. 2016; 22: 221–226.
16.
Nitsch-Osuch A, Kozerska A, Topczewska-Cabanek A, et al. Realization of immunization schedule with recommended vaccines among children from one general practice. Fam Med Prim Care Rev. 2012; 14: 410–413.
17.
Pomian- Osiak A, Owłasiuk A, Gryko A, et al. Vaccination of children at the age of 0–2 with combination and recommended vaccines – assessment of the frequency of use and the knowledge of parents. Probl Med Rodz. 2014; 3: 18–27.
18.
Kędzierska A K. Realizacja szczepień ochronnych u dzieci do drugiego roku życia. Pielęg Pol. 2019; 73: 252–257.
19.
Hubicki L, Czech E, Kowalska M, et al. Szczepienia ochronne dzieci w rodzinach o różnym stanie społecznoekonomicznym w Bytomiu. Przegl Epidemiol. 2004; 58: 713–723.
20.
Gańczak M, Dmytrzyk-Daniłów G, Karakiewicz B, et al. Determinants influencing selfpaid vaccination coverage, in 0–5 years old Polish children. Vaccine. 2013; 31: 5687–5692.
21.
Obando-Pacheco P, Rivero-Calle I, Raguindin PF, et al. DTaP5-HBV-IPV-Hib paediatric hexavalent combination vaccine for use in children from 6 weeks through to 4 years of age. Expert Rev Vaccines. 2019; 18: 1115–1126.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.